A study in mice links estrogen, gut, and bacteria to chronic abdominal pain
A new study with animal models reveals a previously unknown biological mechanism that could explain the higher prevalence of chronic abdominal pain in women. The study, published in the journal Cell Metabolism, identifies how female sex hormones can initiate a chain of events that ends up amplifying pain signals from the gut 🧬.
The hormonal circuit connecting estrogen to visceral pain
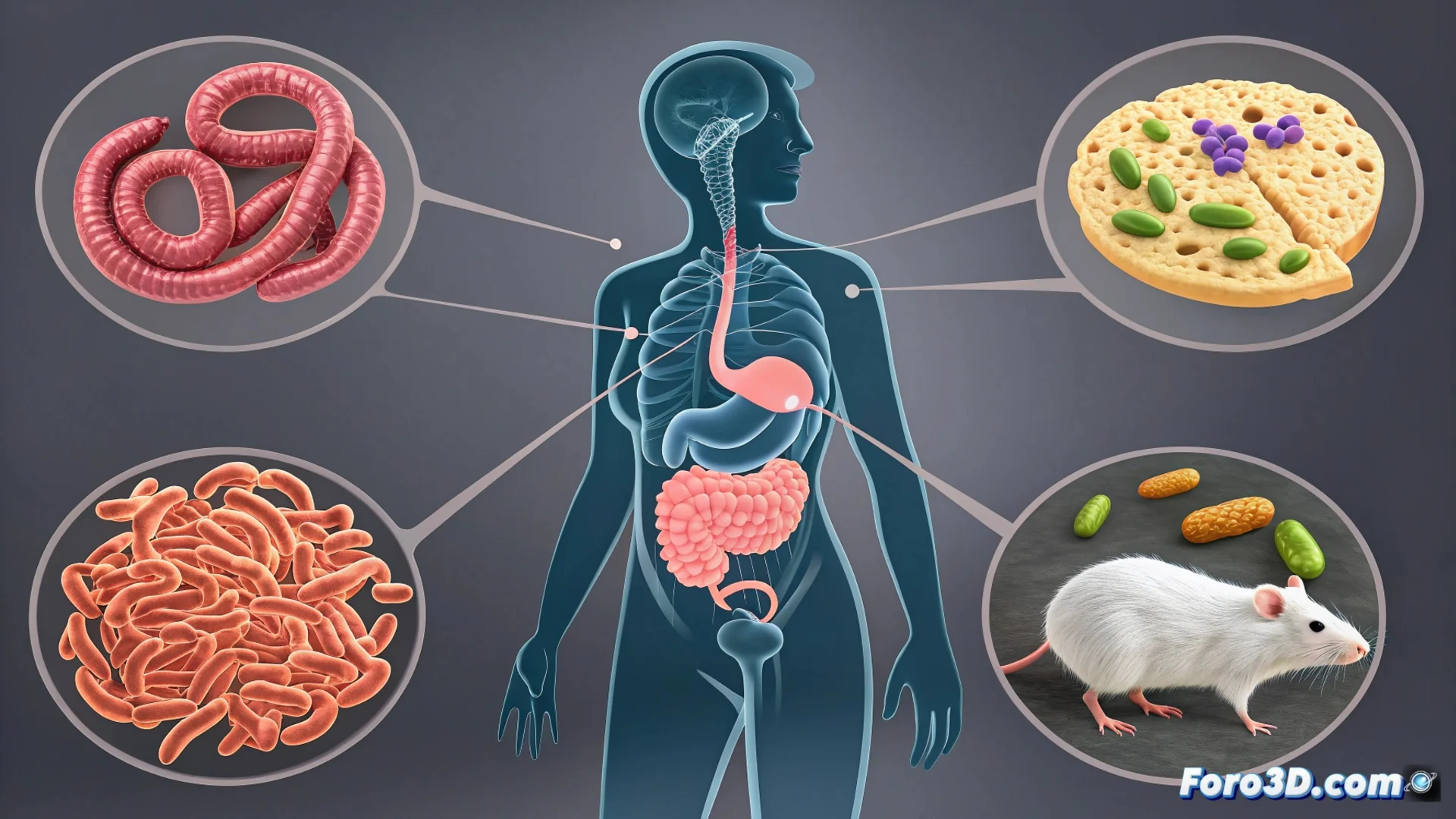
Scientists discovered that estrogen acts as a key switch in this process. Elevated levels of this hormone modulate the activity of neutrophils, a type of defensive cell located in the intestinal mucosa. Upon receiving the hormonal signal, these cells release large amounts of an enzyme called mieloperoxidase.
The triggered biochemical cascade:- The mieloperoxidase oxidizes tryptophan, an essential amino acid from the diet, within the intestinal environment.
- This chemical reaction generates specific metabolites that serve as food for certain bacterial strains.
- Bacteria like Lactobacillus reuteri use these compounds to proliferate, which alters the balance of the gut microbiota.
This estrogen-immunity-microbiota axis represents a direct link between hormones and digestive functional disorders that predominate in women.
From dysbiosis to heightened pain perception
The change in the bacterial population is not an isolated event. The increase in bacteria like L. reuteri leads them to produce more molecules that act as chemical signals. These molecules directly and constantly stimulate the sensory nerve endings embedded in the intestinal wall.
Consequences of persistent nerve activation:- The neurons send more intense and frequent pain impulses to the brain.
- This translates into visceral hypersensitivity, where normally innocuous stimuli are perceived as painful.
- The researchers confirmed the mechanism by blocking the myeloperoxidase enzyme or modifying the diet, which reduced bacterial growth and alleviated pain in the mice.
Implications for understanding disorders like irritable bowel syndrome
This finding provides a solid mechanistic explanation for why conditions like irritable bowel syndrome (IBS) disproportionately affect women. It suggests that natural hormonal fluctuations can, through this axis, modify abdominal pain sensitivity. Science now indicates that gut bacteria, fueled by hormonal signals, may be behind those recurrent digestive discomforts 🔬.