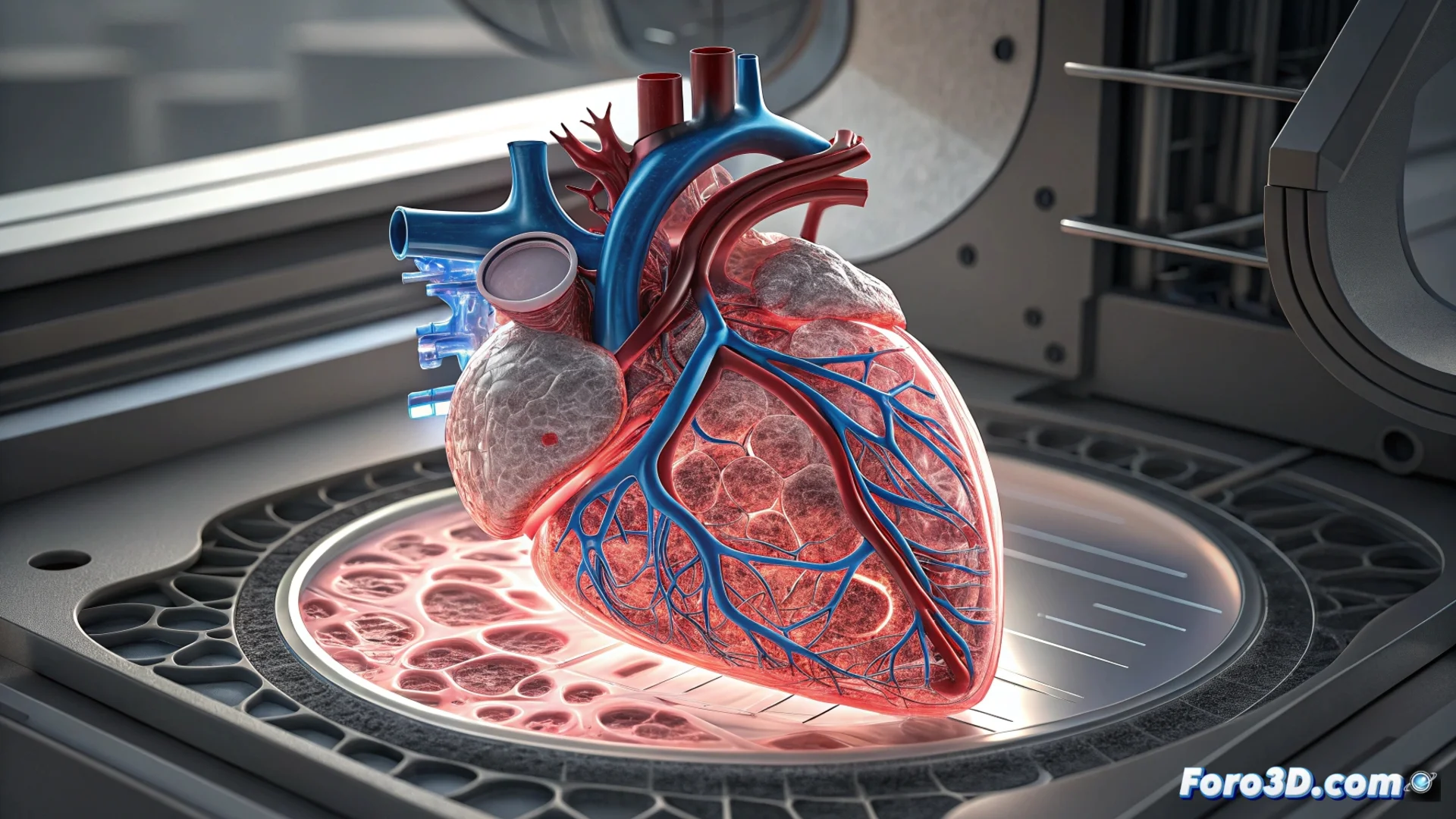
3D Bioprinting Creates Tissues with Functional Vascular Networks
3D bioprinting technology is advancing toward an ambitious goal: manufacturing complex human tissues that incorporate complete vascular systems. These internal channels, which mimic veins and arteries, are essential for blood to flow and nourish every cell in an artificial organ. Without this network, cells in the center of the tissue die from lack of oxygen, making vascular integration the decisive step to achieve viable transplantable organs. 🫀
Strategies for Printing Blood Vessels
Scientists employ several techniques to build these microscopic tubular structures. A common method uses sacrificial scaffolds that, once printed, dissolve to leave hollow spaces that become channels. Injection bioprinting is another strategy, where cells and a support material are deposited simultaneously to define the vascular architecture. More advanced approaches incorporate endothelial cells, which naturally form the inner lining of vessels, allowing them to self-organize and generate stable tubes within the printed tissue.
Main Vascular Biofabrication Methods:- Fugitive Scaffolds: A structure is printed from a material that is later removed, leaving a network of hollow channels ready to be colonized by cells.
- Simultaneous Injection Printing: Layers of cellular bio-ink and a support hydrogel are deposited in a coordinated manner to create integrated conduits.
- Guided Cellular Self-Assembly: Endothelial cells are seeded in specific patterns to migrate and form vessels naturally.
Integrating a functional vascular system is the major bottleneck in moving from printing tissue patches to generating complete organs.
Obstacles to Manufacturing Complete Organs
Overcoming the creation of microvessels is just the first step. The main challenge is connecting that printed network to a patient's circulatory system. The artificial vessels must be robust enough to withstand constant blood pressure without breaking or leaking. Additionally, it is crucial that the various types of cells in the organ, such as those in a liver or heart, integrate and communicate correctly to perform their specific function. Scale also poses a problem, as printing an organ the size of an adult kidney requires extreme precision and a very long manufacturing time.
Critical Pending Challenges:- Vascular Connection: Linking the bioprinted organ's microvasculature to the recipient's arteries and veins.
- Functional Integration: Achieving cooperation among all cell types so the organ filters, pumps, or secretes like a natural one.
- Immune Response: Convincing the body's immune system to accept the printed organ and not identify it as a foreign body to reject.
The Limit Beyond the Printer
Perhaps the most complex challenge does not lie in the 3D printer or the bio-inks, but in the recipient's biology. Even the best-designed organ must avoid triggering a rejection response from the immune system. This is a problem that no printing technology, no matter how advanced, can solve by simply pressing a button. It requires parallel advances in immunology and regenerative medicine. The path to printed replacement organs is, therefore, multidisciplinary. 🔬